Effluent Quality Standards For Paper Mills

Volume 6, Issue 1, January-March 2023
Effluent Quality Standards For Paper Mills
Shamim A. Rayani
Assistant General Manager -HSE, Kanpur Fertilizer & Chemical Ltd, Kanpur (UP)
Email id: shamim.rayani@gmail.com
Abstract
Water serves a variety of purposes just on planet and is among the most fundamental ingredients of life. Erosion of soil or rock by flowing water results in the movement of suspended, colloidal and dissolved constituents over great distances, often thousands of kilometers, before they are ever redeposited.
Because of the integration of human activity into the movement of material within natural waters, the contamination of these processes is rising. In order to reduce the amount of pollution load delivered to receivers, several treatment procedures are used. Water cleansing takes a long time in the natural world. Naturally occurring phenomena are imitated by man-made methods of treatment, but at a far faster pace than natural forces. Mechanical, physiological, chemical, and biological processes are all examples of methods of treatment that may be used alone or in tandem. Processes for wastewater treatment involve both conversion and separation operations. Bacteriological mechanisms or chemical precipitation into submerged and/or dispersed substances change the liquid and/or liquid components into dusty solids during processing. Clogging, filtration, and flotation are all methods of removing suspended compounds from water after they have been suspended.
The introduction of oxygen in into water, mixing, transporting, collecting, and lifting are all activities that need power to be accelerated. Therapeutic periods are reduced to less than 12 hours when such expedited procedures are used. Most conventional treatment approaches are inadequate by themselves, however, due to the high financial costs associated with these procedures. It is vital to conserve our environment and climate from further degradation, particularly for the sake of the survival of certain threatened animals. It is common practise to dump waste into the seas, streams, including lakes that are really the lifeline of many regional enviro, causing harm to whatever is reliant on these water supplies.
KEYWORDS: Waste Water, Paper Mill, Effluent, Water Pollution, Environment, Safety etc.
1. INTRODUCTION
The Indian paper industry is distinguished for its range of products. The production capability of the Indian paper mills ranges from 15 to 1500 tonnes per day. A broad range of raw resources are used in the Indian paper mills, which makes standardisation of the manufacturing process challenging. The industry employs a diverse range of technology, which differs across sectors including within industries. The development of resource efficiency, maintaining global competitiveness, managing with fibre scarcity, and resolving environmental concerns and difficulties are the most significant challenges confronting the Indian paper mills sector in the next years. Despite the fact that the Indian service sector has implemented a number of projects aimed at technological advancement and pollution prevention initiatives in recent years, performance indicators including such thermal efficiency, specific rainwater harvesting, and particular effluent generation have all improved. However, concerns of technological obsolescence as well as a lack of standardisation in the manufacturing process must be discussed urgently in task phase in order to paper industry to continue to grow in a sustainable way in the new group of protecting environment and in the face of global competing.
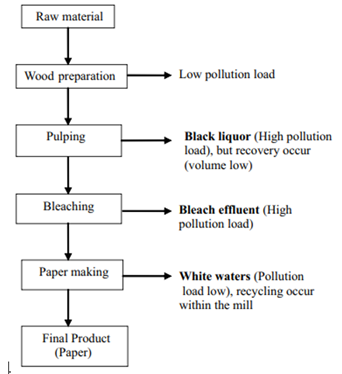
Figure 1.1: Wastewater sources in pulp & paper industries
2. Environmental Impacts of Industrialization
Industrialization (also known as industry) is a process that takes place in nations when they begin to employ technology to do tasks that were previously performed by humans. As urbanization progresses, it alters the social landscape. When a nation undergoes industrialization, people leave their rural occupations in order to seek higher-paying positions in industries in urban areas. Industrial growth is a stage in a process through which people embrace simpler and less expensive methods of producing goods. It becomes feasible to manufacture more items in a shorter period of time when greater technology is used to do so. A single individual may generate more than one item. People’s occupations become increasingly specialised as a result of industrialisation. For example, prior to industrialisation, a shoemaker was responsible for the entire shoe. He began by working solely on a single pair of shoes, completed that set, and then moved onto next pair of sneakers. As a result of industrialisation, there are a large number of individuals employed in the shoe manufacturing industry. An single shoemaker, on the other hand, has a more limited scope of work. There is one worker who is in charge of cutting the soles of the shoes. It is then stitched on by another individual. Briefly stated, there is a division of work. Because the machinery used to manufacture the shoes are expensive, the factory would be owned by a wealthy individual who can afford to purchase the equipment.
Mechanisation, in the name of economic development, has placed enormous burden on the environment. In emerging nations, industry and environmental protection are attempted to go hand in hand. However, whether consciously or unwittingly, industrialization outran the competition by running faster and with less regard for the environment. During the last year, the rate of industrialisation has grown by orders of magnitude. There is a good chance that the majority of the world’s most serious environmental issues in the twenty-first century will be caused by the continuance and escalation of existing issues that are presently receiving insufficient political focus. In many nations, issues are either not observed at all or are not addressed at all even after they have been identified. Changing climate, freshwater shortages, deforestation, water pollutants, and population increase are among the most pressing concerns facing the world today. These issues are very complicated, and it is difficult to determine how they interact with one another. It is essential to consider issues in the context of social system as a whole. Even while the links between ecological problems are now well understood, we still lack precise knowledge on how the issues are related, to what extent they communicate, what the most successful interventions are. One issue is how to combine land- and moisture management in order to ensure food and water security for everyone. During the previous two decades, rapid industrialisation in order to fulfil consumer demand has wreaked havoc on the ecosystem to the greatest degree possible. In addition to being a threat to human health and the environment, industrial effluents, contaminated air, noise and vibration, and the Green House gas impact, among other things, are a source of worry for future calamities. For the sake of living a healthy life, we are degrading our surrounding environment inside the background.
Worldwide, as previously said, industries have become more concerned with attaining and exhibiting their environmental and social performance in order to comply with more stringent regulations and to respond to increased public demand.
Natural problems such as ” the Bhopal tragic incident, Rhine river pollution, Chernobyl disaster, acid rain damage, and ozone layer depletion have increased public pressure on governments around the world, which has resulted in governments around the world enacting stringent environmental legislation with heavy punishments in environmental problems related to environmental and safety systems” .There are no particular environmental important role in affecting specified in these standards; rather, they are system norms that describe the planning of the environment in accordance with the company’s climate issues as well as objectives and targets that have been defined in accordance with their considerable environmental impacts.
Due to rising compulsions from rigorous regulation and rising public criticism, industry has become more concerned about attaining and exhibiting solid sustainability impact. When the ecosystem was harmed by urban and animal activity, hardly one seemed to care. That was not so long ago. In addition to affecting the living conditions, pollutants have an impact on the social, economic, political and aesthetic of a society. In latest days, there has been an increase in public awareness of the dangers of environmental contamination.
On the one side, scientific and technological breakthroughs have improved human pleasures by providing us with automobiles, electrical items, better medications, and better chemicals to control damaging insects and pests, though on the other side, they have created a very severe problem in the form of environmental pollution. Continuing pollution growth, along with the industrialization, has had a significant influence on natural resource depletion and exploitation. Financial development’s long-term viability is jeopardized by the environment degradation and the rapid resource depletion that has resulted. Developing a feasible synthesis between trade and environmental behavior is one of the most serious and complicated concerns that our generation faces today.
2.1 Industrial Water Pollution
A significant cause of water contamination, industry generates chemicals that are exceedingly hazardous to both humans and nature. Freshwater is used by many industrial plants to transport waste away out from facility and into rivers, ponds, and seas, among other places.
In the case of water contamination, it occurs as a result of the release of dangerous chemicals and compounds into water, rendering the water unfit for drinking and other uses. This makes the water unusable for people and puts aquatic life in peril as a result.
Pollution is defined as the environmental contamination by detrimental and solid waste, which results in a significant deterioration in the composition of the outer air, hydrosphere, biosphere, land surface, and, increasingly, the performance of the surroundings. Pollution can occur anywhere on the planet. In the context of water pollution, it refers to the contamination of water, rendering them unsuitable for drinking purposes. Despite the fact that water covers 70% of the Earth’s crust, the freshwater in the ocean waters is salty and, as a result, cannot be utilised for drinking, agricultural, or industrial purposes. Fresh water can only be found in bodies of water such as lakes, ponds, canals, aquifers, and streams.
Many chemicals are used in agricultural and manufacturing activity, and these chemicals may leach into the groundwater and damage it. Steel mills and other industrial operations damage rivers and lakes with heavy metals and chemicals. Aquatic life is harmed and rendered sterile as a result of these pollutants. Pesticides are being used to keep weeds, pests, and fungus under control. The aquatic life is poisoned by the chemicals that run off into the environment. The consumption of diseased fish by birds, people, and other animals has the potential to cause poisoning. Petroleum is a separate form of biological pollutant that may contaminate water if a ships ruptures, resulting in oil spills into the ocean. Oil spills have a localised impact on animals, but they have the potential to spread hundreds of kilometres. This oil has the potential to kill a large number of fish and adhere to the hair of birds. As a result, they lose their capacity to fly.
Pollution occurs when silt as well as other particles, such as soil, building materials, and runoff from ploughed lands, enter rivers and streams. In lakes, canals, and other bodies of water, nutrient enrichment happens naturally under certain circumstances. This is a normal aging process that causes silt and organic materials to accumulate in the water body. As a result of these sediments entering diverse bodies of water, fish breathing is hampered, while plant production and water depth are also reduced.
2.2 Industrial Effluents in the Water
Water pollution is produced by the discharge of home or urban wastewater, agricultural runoff, contaminants, and industrial discharges into waterways, among other things. Nowadays, the debris emitted by industrial units serves as the primary source of contamination. The waste products produced by many production plants, including acids, alkalies, hazardous metals, oil, grease, dyes, insecticides, and even radiological compounds, are disposed of in water bodies and rivers. PCB combinations, lubricants, and water heating produced by power plants are some of the other major pollutants to be concerned about. In most cases, the contaminants that are discharged into bodies of water decompose or stay submerged in water. They can also build on the bottoms of lakes and rivers from time to time. Oil spilt from oil tanks is yet another significant contaminant that has the potential of affecting marine life. On average, 1.3 million barrels of crude oil are dumped into Persian Gulf each year, and around 285 million gallons of diesel are poured into the seas each year, according to estimates from the United Nations. In addition to mesothelioma, phosphates, phenols, pesticides, toxic colours and other harmful elements, industrial effluents also include mercury, copper, nitrates, sulphur, sulfuric, oil, and a variety of other hazardous substances. In many nations, commercial water is not thoroughly cleaned before it is discharged into rivers and lakes, resulting in water pollution. This is especially true of small-scale enterprises which do not have the financial resources to invest in antipollution technology.
2.3 Organic Pollution Due to Industrialization
2.3.1 Organic Pollutants:
Persistent organic pollutants (POPs) are harmful compounds that have a negative effect on public health and the ecosystem all over the globe, including the United States. Because they may be transferred by wind or water, the majority of POPs produced in one nation can and do have an impact on humans and animals in other countries where they are utilised or discharged. They may remain in the environment for long durations and can collect and spread between one species to another along the food supply chain. POPs are compounds that stay in the ecosystem, micro via the food chain, and represent a hazard to public health and the ecosystem by creating detrimental consequences. As a result of the proof of long-distance travel of these materials to areas for which they have not been used or generated, as well as the dangers they face to the environment, the world community has already called for immediate global measures to stop and remove the discharge of these contaminants on a number of different occasions.
- Highly Toxic to human & the environment
- Persistent in the environment, resisting bio-degradation
- Taken up and bio-accumulated in terrestrial and aquatic ecosystem
- Capable of long-range, trans boundary atmospheric transport and deposition
These compounds have an impact on the development & growth of plants and animals in the wild. They have been linked to decreased reproductive fitness, birth abnormalities, behavioural disorders, and even mortality in certain cases. They are probable human carcinogenic, and they have the potential to affect the immunological and hormonal systems as well.
Surfactants are widely used in a variety of sectors, including textiles, fibres, food, paints, plastics, cosmetics, medicines, mining, oil reservoirs, pulp and paper, and laundry detergents, dishwashing solutions, and shampoos, among others. Surfactants are also used in a variety of industrial applications, including lubricant, polymerisation, textile manufacturing, mining mean working, hydrocarbon recovery, sewage treatment, and a variety of other goods and services. Harsh detergents are often used as absorbents in the aftermath of an oil spill. Dozens of chemicals may be employed as surfactants, and they are often classed according to their electrical activity in solutions: anionic, charged, non-ionic, or amphipathic. Each surfactants class does have its own set of characteristics that distinguish it from the others. Various sources of detergents are released into natural waterways, and each has its own unique composition. Textiles, surfactants, and detergent formulation are examples of industrial sources. Harsh detergents are also utilised in the laundry and in the home, and as a result, they may be discovered in the effluent from wastewater treatment facilities. Pesticides, property of a set, and dispersants are some of the agricultural uses for these compounds.
Surfactants are organic molecules that have both hydrophilic and lipophilic or lipophobic groups in their structure. owing to the fact that they have both a hydrophilic and a chemical inertness, surfactants tend to focus at the integrations is something; the water – soluble part of cleaning agent tends to orient itself towards the aqueous medium and the hydrophobic tail of the foaming agent tends to orient itself apart from the aqueous solution into the phase
When it comes to surfactant molecules, the wet portion is often formed by distilling a hydrocarbon comprising 8 to 20 carbons (e.g. fatty acids, paraffins, olefins). In aqueous systems, the hydrophilic fraction may ionise (become cationic or polyatomic) or may stay un- ionized (non-ionic).
Solvents are responsible for formation of foams in streams and wastewater treatment facilities, as well as for lowering the overall water quality. Detergents are responsible for changes in the environment. When skin is exposed to surfactants for an extended period of time, the lipid layer that covers skin (as well as other) cells might be disrupted. The presence of surfactants in groundwater, such as bodies of water, may result in a detrimental scenario for aquatic biota, owing to the fact that they may interfere with oxygen transport by altering surface tension, resulting in a reduction in transfer of oxygen. Another potentially harmful aspect of these items is their tendency to bind with other harmful toxins (such as medications), which has the potential to disrupt ecological quality
Dyes are among the most common ingredients of wastewater created by a wide range of businesses, including textile, paints and varnish, ink, polymers, pulp and paper, skincare, tannery, and other related sectors, as well as by dye manufacturing companies themselves. Water contamination as a result of effluents from the dyeing and finishing industry is a major source of public concern. Color is a significant component of the human experience. We like wearing clothing in a variety of colours and hues, eating food that has been coloured, and even taking medications that are brightly coloured. Therefore, it comes as no surprise that a great deal of study went into the manufacture of colour. Today, there are over ten thousand colors available for purchase on the market, with seven lakh tonnes of dye being manufactured yearly. Dyes are available in a variety of structural types, including acidic, base, dispersion, azo, affects adversely, and metallic ions dyes, among others. Acidic, simple, and dispersion dyes are among the most common. The textile sector is the world’s biggest user of dyeing materials and products. An extremely substantial proportion of the dyestuff does not bond to the textile fiber and is discarded into the waste stream during the colouring process. Around 10-15 percent of the dyes used in the dyeing process are discharged into the atmosphere, resulting in effluent that is highly coloured and aesthetically objectionable. Toxic residues from various sources (for example, textile factories, pulp & paper businesses, dye and dye intermediates businesses, pharmaceutical industries, leather tanning and Kraft decolorization industries, and such.) are known a wide variety of environmental substances that have been introduced into water sources as well as treatment systems, according to the World Health Organization.
Environment-related difficulties associated with residue left dye subject matter or leftover colour throughout treated textile industries are always a source of concern for each fabric operator which thus directly discharges, including sewage treatment plants and advertising textile operational processes, in terms of adhering to the colour as well as lingering dye requirements imposed on treated textile wastewater discharges. High levels of dyestuff in water bodies reduce the reoxygenation ability of the receiving stream and block out sunlight, disrupting the bioactivity of aquatic life as well as the photosynthetic process of aquatic species or algae, according to the Environmental Protection Agency. With dyes, the most significant environmental problem is their absorbance and reflections of sunlight into the water, which results in a decrease in photosynthesis as well as the concentration of oxygen in river. In contrast, certain colours breakdown into chemicals that are poisonous, mutagenic, and cancerous to living organisms when exposed to them. Consequently, the wastewater from textile manufacturing facilities contains a huge variety of dyes as well as other chemicals that are applied during the dyeing and colouring procedure. In typical water treatment techniques, they are hard to remove, and they’ll be carried readily via drains and rivers, particularly since they are engineered to have great water solubility. As a result, dyes have the potential to be harmful to living creatures. As a result, it is critical to protect the ecosystem against such toxins.
3. CONCLUSION
“Electrochemical wastewater treatment can be recommended as a feasible alternative for pulp and paper wastewater mitigation based on findings from an experimental setting. With respect to the decrease of organic load and colour of wastewater, the electrolytic approach that was tested produced good results. – For the paper industry’s effluents, it should be underlined that colour reduction is of critical significance. Percentage reductions in COD and colour are almost equal to or more than 80 and 90, respectively. A viable tertiary treatment option for the paper industry, owing to the simplicity of the electrochemical approach, may be water treated by electrochemical technology, which would not present any problems in terms of recycling of the treated water To take care of the inorganics while also lowering the cost, the approach may be improved even further via optimization.
REFERENCES:
- Alexandersson T. (2002). Time variation in whitewater composition from two recycled paper mills. TEIE-7181, Dept. of Industrial Electrical Engineering and Automation, Lund University, Lund, Sweden, 2002.
- Awa Instruments SAS, Meylan, Zirst, France (2003). www.awa- instruments.com (April 22, 2003).
- Barascud M. C., Ehlinger F., Pichon M. and Ruoger J. (1992). COD removal in a closed water circuit of a papermill by an anaerobic fluidized bed reactor. Wat. Sci. Tech., 26(1-2), 445-454.
- Barnett D. J. and Grier L. (1996). Mill closure forces focus on fines retention, foam control (Part 3). Pulp & Paper, 70(4), 89-.
- Berard P. (2000). Filling in the holes after closing the loop. Pulp and Paper International, April, 44-51.
- Blanco M. A., Negro C., Gaspar I. and Tijero J. (1996). Slime problems in the paper and board industry. Appl. Microbiol. Biotechnol., 46(3), 203- 208.
- Boyko J., Anderson J. and Lockhart C. (1999). Reduction of paper machine water consumption – Significant savings can be made. Pulp & Paper Canada, 100(7), 42-45.
- Brock T. D. and Madigan M. T. (1991). Biology of microorganisms. Prentice Hall International Ltd. London, UK.
- Buchanan B. (1980). Closeup of whitewater system helps Menasha meet effluent standards. Pulp & Paper, March, 60-62.
- Charpentier J., Florentz M. and David G. (1987). Oxidation-reduction potential (ORP) regulation: A way to optimize pollution removal and energy savings in the low load activated sludge process. Wat. Sci. Tech., 19(3-4), 645-655.
- Charpentier J., Godart H., Martin G. and Mogno Y. (1989). Oxidation- reduction potential (ORP) regulation as a way to optimize aeration and C, N, and P removal: experimental basis and various full-scale experiments. Wat. Sci. Tech., 21(10-11), 1209-1223.
- Chaudhary A., Gupta L. K., Gupta J. K. and Banerjee U. C. (1997). Studies on slime-forming organisms of a paper mill – Slime production and its control. J. Ind. Microbiol. Biotechnol., 18(5), 348-352.
- Danadurai, K. S. K. and Rajeswari S. (1999). Corrosion behaviour of 316L stainless steel and titanium-stabilized stainless steels in a paper- machine white-water system. Corros. Prev. Control, 46(2), 39-45.
- Danfoss Analytical A/S, Sønderborg, Denmark, (2003). www.danfoss.com/analytical (April 22, 2003).
- Degrémont. (1991). Water Treatment Handbook. Lavosier Publishing Inc., Secaucus, USA.
